Recently, the sketch above got a bit of attention on Twitter when it was re-discovered by Matteo Farinella, a Ph.D. scientist by training and comic artist who has studied the unique ability of comics to communicate science. He couldn’t believe that he’d stumbled on an honest-to-goodness scientific figure made in the style of a comic. He would have recognized techniques I used from Scott McCloud’s much-revered book, “Making Comics”, to attempt to give a sweeping historical overview of molecular glues in a very small space for an article called “Rise of the Molecular Glues” by Stuart Schreiber.
I couldn’t believe I’d stumbled on treasure troves of science comic artists and also research into the role of art in science—and not just communicating science once the results are in. Here were people studying how art can be instrumental in the process of doing science.
The art of comics is not confined to the visual. Researchers can lean on narrative and metaphor inherent in the medium. In Farinella’s own 2018 review of many of these studies, he concedes that more unbiased and carefully controlled studies are needed, but that, “while comics are often perceived as an easy and playful format, they may be exquisitely suited at presenting complex information in a rigorous yet accessible way.” Perfect for looking at sequential processes at multiple scales.
If the image above was more than a single figure of restricted dimensions in a journal, it might have been coupled with a metaphorical narrative. In this post, I explore the use of metaphor as an artform to explain nuances in molecular glues, and then dig deeper to explore how metaphors like this could be used by researchers to expand their thinking and inspire new experiments.
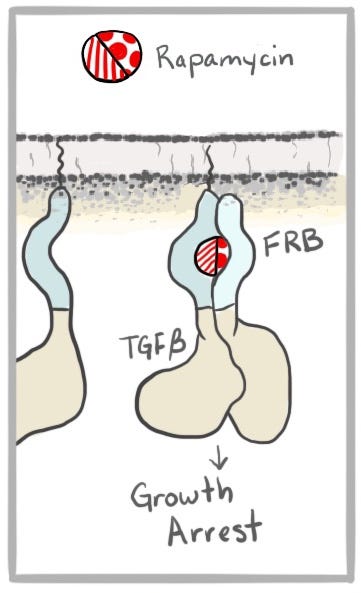
Before anyone knew what a molecular glue was, the molecules cyclosporin, FK506, and rapamycin, exquisite products of billions of years of evolution, were wiling away their days underground and in complete obscurity in fungus and soil bacteria. The discovery in the 1970s that cyclosporin suppresses the immune system thrust it into the limelight as the hero that made organ transplantation possible by preventing rejection of a donor organ by the host’s immune system. When FK506 was later shown to do the same but better and with fewer side effects, it became the darling of a small fledgling biotech company called Vertex in the late 80s to early 90s, and a main character in Barry Werth’s 1995 book, The Billion Dollar Molecule. It also reminded Vertex co-founder Stuart Schreiber of a molecule he’d seen before called rapamycin, known for its anti-cancer promise.
Schreiber’s lab connected the dots for the first two in 1991, and for rapamycin in 1994, discovering that they fulfilled their functions by encouraging proteins to stick together. He called them molecular glues. In a 1992 article called Molecular Matchmakers, Brandeis professor Dagmar Ringe called them “schatchens”, which is a Yiddish word that means marriage broker. Both metaphors are apt. They are small molecules that create a marriage between proteins by making them stick together. But today we distinguish molecular glues from bifunctional molecules, the latter being more like marriage brokers, and I’ll explain why.
To make the analogies comparable, we can think of a molecular glue as Cupid’s arrow. With its roots in Greek mythology, a simplistic modern-day summary is that when a person gets shot with the cherubic Cupid’s arrow, they fall hopelessly in love. When a molecular glue becomes lodged into its target protein, that target protein becomes irresistibly attracted to (and attractive to) another specific protein—one it would have innocently snubbed only moments earlier.
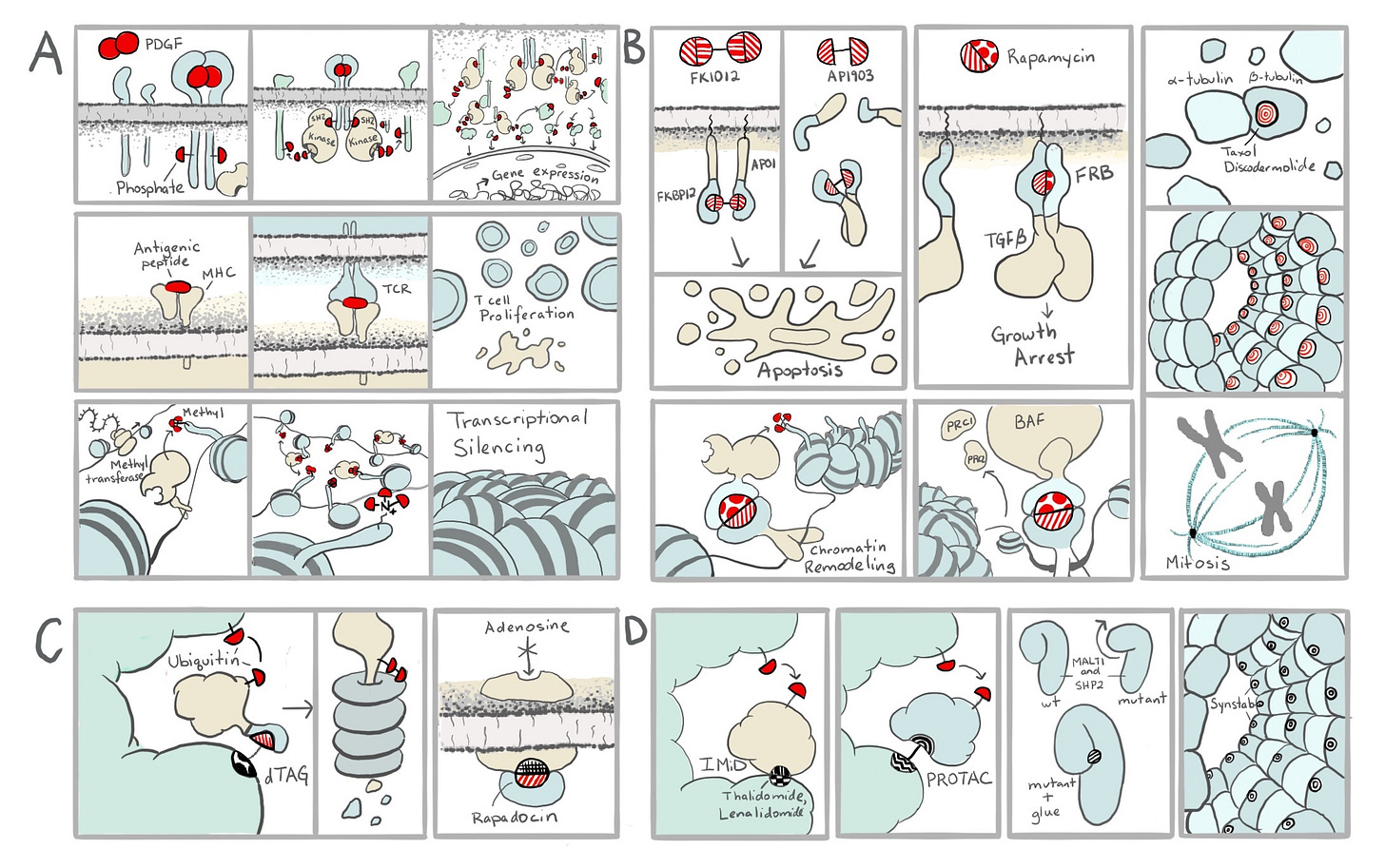
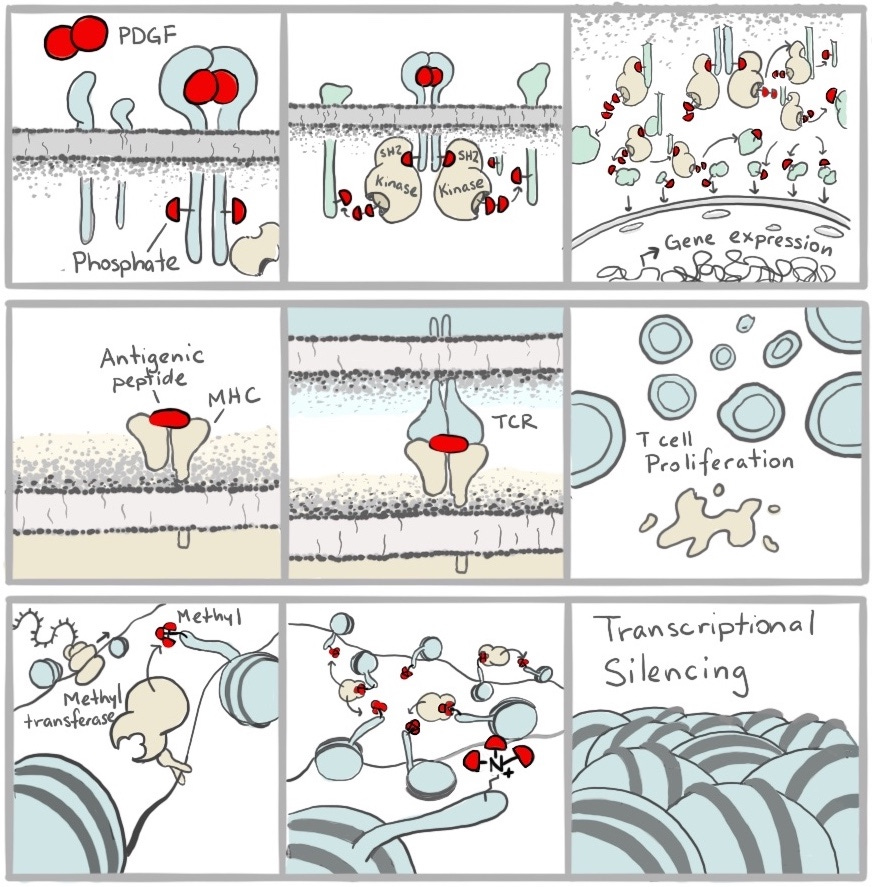
After the Schreiber Lab discovered the amorous potential of these molecules, they immediately recognized an opportunity. Many natural biological processes are driven by proximity. This is not an abstract concept. Everything you see in the picture below is happening in our bodies right this second as you read this. Information is being passed from the outsides to the insides of our cells, our immune systems are dispensing of threats we’ll never need to know of, and our genes are being turned on and off, all because the right proteins are being seated next to each other.
To realize the opportunity, Schreiber teamed up with Gerald Crabtree at Stanford in what would be the beginning of a magical collaboration and a lifelong friendship. They figured out exactly how two of the molecular glues blocked the immune system, and realized that they could use them to make other things happen too. They could bring all sorts of proteins together by attaching them to the protein targets of molecular glues. In these cases, the couple brought together by Cupid’s arrow brings along their best friends in hopes they will hit it off. The relationship between the tagalongs—tethered as they are to their hopelessly lovesick friends—generally remains transactional. But that doesn’t matter to the cell, which doesn’t know the difference. With these tools, they didn’t just make things stop working—they could make them start working too, like tricking cells into turning on genes and performing other such feats like the flip of a switch.
The surprisingly robust success of these experiments by Schreiber and Crabtree captured the imagination of an entire field, coined chemical biology because small molecules were being used deliberately to control biology. The best friend approach requires genetic engineering, so chemical biologists over time began to think beyond Cupid, designing molecules that acted like old-timey matchmakers, or schatchens. All they needed to do was take a small molecule that sticks to one protein and attach it to a small molecule that sticks to another protein. The resulting creation, called a bifunctional molecule, would bring the proteins together. The mates didn’t need to have any affinity to one another. They only needed to be brought into close proximity. Matches were made simply to enable a favorable outcome, be it inhibiting, activating, stabilizing, destabilizing, or relocating one of the proteins, to name just a few.
One of the many, many spinoffs that is getting a lot of attention these days is the use of matchmakers to target toxic proteins for destruction. This particular brand of matchmaker connects a secret agent to a nefarious character. The agent surreptitiously drops a device in the villain’s pocket, targeting them for later destruction (the destruction being analogous to a scene at the end of the movie Fargo, if you must know).
More recently, researchers have stumbled on Cupid’s arrow-style molecular glues like thalidomide and lenalidomide with similar assassination potential, raising the possibility that perhaps they aren’t the ultra-rare evolutionary marvel they were thought to be. Maybe we could find more.
For many reasons, these true molecular glues are often preferable to bifunctional matchmakers. One of the main differences between the true love of Cupid’s victims and matchmaker-brokered marriages of convenience is cooperativity. I don’t mean sharing the chores (I want you to want to do the dishes!). On the molecular level, this kind of cooperativity produces a mathematical Hill coefficient of >1. That just means that when the protein gets impaled with Cupid’s arrow, its affinity for its beloved grows. Not so in the marriage of convenience.
Another complication of the marriage of convenience is that if the market for matchmakers becomes saturated, two people may not be coupled because each already has a matchmaker that doesn’t want to share the commission. In molecular terms, this is called the hook effect. If both proteins are already occupied separately by bifunctional matchmakers, they can’t be brought together. So at a certain concentration, matchmaking fails. Cupid’s arrow of the molecular glue, on the other hand, impales only one of the proteins, and then together they engage the other one. No hook effect.
Rather than waiting around for Cupid to shoot another arrow, we now have more tools. Diversity-oriented synthesis and large-scale screening are ways to make and test legions of new molecules—any of which could be a molecular glue. Together they are like dating apps. With such volume and speed, it’s never been easier for potential soul mates to find each other.
But what about that picky friend who never ever swipes right? They are what’s known in the drug discovery world as the undruggable protein. Many scientists believed that there was no hope for these perpetual molecular bachelors. What could we do if no molecule will stick to the protein we want to target?
As we sit on that question for a minute, let’s consider the metaphor before we get too carried away. No metaphor is perfect, so we need to understand where they fail. As Farinella warns, “it is important to consider the role of fictional characters and the use of anthropomorphism in comics, which may facilitate readers’ engagement with scientific subjects but also potentially promote a false sense of understanding.”
For example, here’s one problem with the metaphor I’m using here. Love between humans is not always mutual. If molecular glues are Cupid’s arrows, they engender equal love in the impaled and their beloved. Likewise, whatever attraction or repulsion is felt or not felt between proteins coupled by matchmakers is also always mutual. So if I led people to believe that proteins could act like love-sick suitors, chasing helpless proteins around like Pepé LePew, that would be useless at best, and dangerous at worst. So these metaphors need to be employed carefully and with appropriate caveats.
Another problem is that a single dating app presents a plethora of potential matches. There are a handful of dating apps, but basically this is a fixed variable. In contrast, current screening efforts focus on varying the candidate molecular glues against fixed proteins, like one person trying every available dating app in hopes of connecting to the singular object of their affection. If there were as many different dating apps as there are candidate molecular glues in a screen, there’s a good shot this approach might work. But that got me wondering whether one could also vary the potential protein recipients of Cupid’s arrow to increase the chances of finding a combination that would bind the target protein. Schreiber responded, “Desirable but technical challenges.” Oooooh, scientists love a challenge!
Matchmaker versus Cupid’s arrow makes a decent communication tool with caveats, but how can it also be used as a research tool, prompting new questions about the science? Maybe we can we use what Evelyn Fox Keller calls “narratives that make productive use of the imprecision of metaphor” in her book, Making Sense of Life: Explaining Biological Development with Models, Metaphors, and Machines. Digging deeper into the example of the undruggable protein/pickiest friend, it’s worth pointing out that if your friend is not in the right place mentally to be in a relationship, nothing you throw at them will stick. Maybe they’re not picky. Maybe they’re just not ready.
Can proteins be put in the right “frame of mind” to be more open to love? There is no point in engineering non-physiological conditions to get a match that wouldn’t be replicated in a real human, but patients harbor a variety of conditions: an oxygen-starved tumor, stress-induced conditions that cause proteins to change shape and huddle together, cells that rearrange their interiors to resist chemotherapy. What if a molecular glue worked only in one of those conditions? That would be something.
I had so much fun exploring this metaphor that I had to cut nearly twelve hundred words from this post (you’re welcome). I didn’t even get into the polyamorous relationships in which glues bring together more than two proteins, or intramolecular, single-protein glues (“I’m just going to date myself for a while”). Readers familiar with molecular glues (and arranged marriages) will find holes just as I did. But can you peek through the holes to find a vista you hadn’t seen before?
I would love to hear about it if you try this exercise for your own work, scientific or otherwise. And if there is anyone you know who might be interested in these explorations, be they your students, postdocs, labmates, coworkers, friends, neighbor who watches a lot of sci-fi, etc., please consider sharing below. Thank you!






Mary, I saw it on LInkedIn — which made me realized that I’d thought that I’d previously subscribed — but apparently hadn’t. I just read today’s piece — and it’s great! Even with all my Broadie years and ancient biology degree, I tend to skim the details. What you published today about thalidomide nailed a no-skim rendering. I don’t know anyone writing about this subject the way you do. It’s so refreshing!
Mary, you are brilliant! What a treat to read.