Making Lemonade
A Random Walk, an 8-Year-Old Drawing, and a Bit of Hope
I didn’t intend to write about artificial photosynthesis this month. But what I wrote first didn’t say anything that people with much bigger megaphones weren’t already saying. So I chucked it. Then I took a walk.
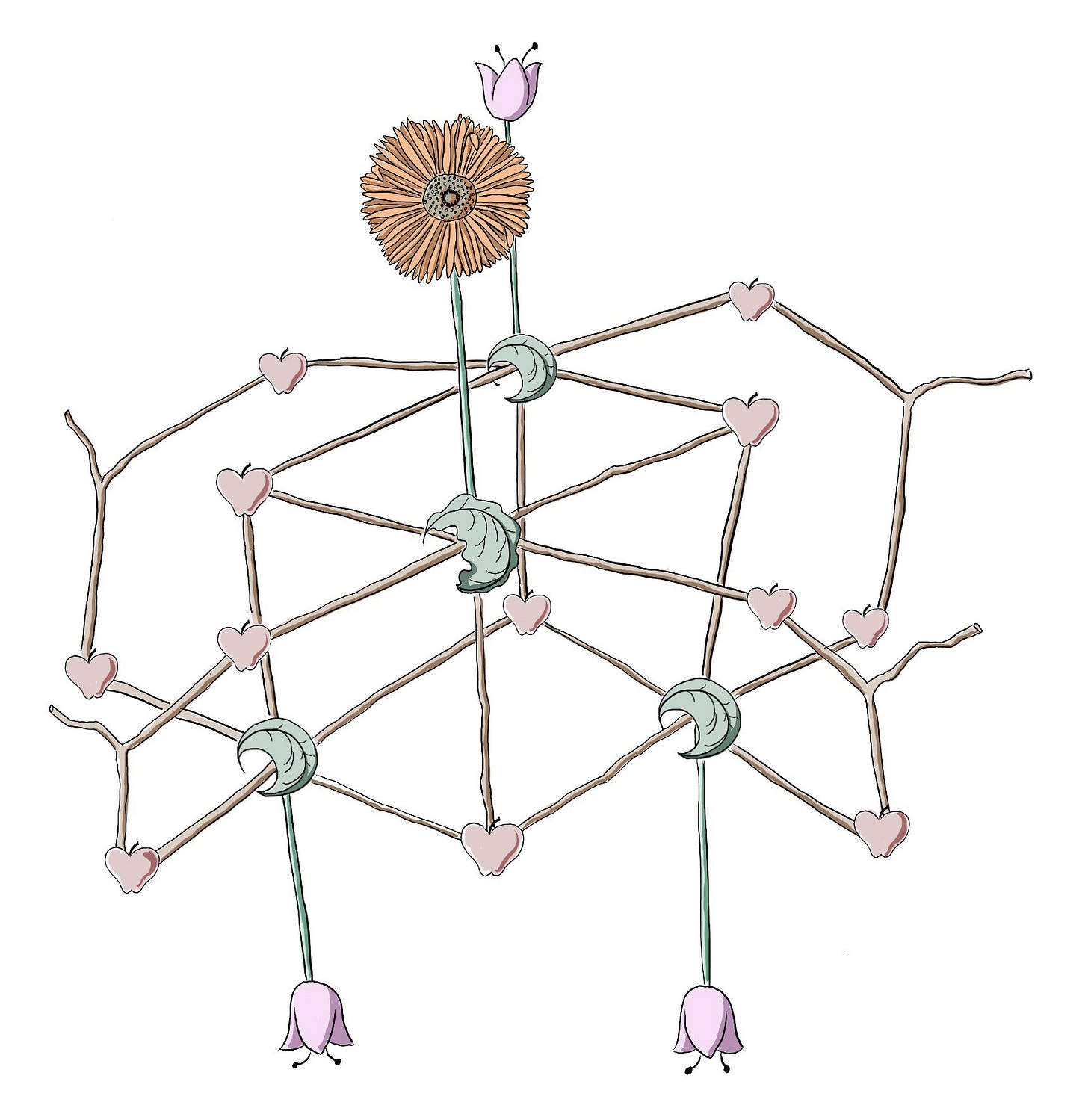
I noticed a shrub that was still bare but for its yellow flower buds, straining against captivity, gasping for the sun, and I remembered a drawing I did in 2017. It represents the structure of a cube-shaped molecule with cobalt (flat-edged leaves) and manganese (serrated leaf). It was synthesized to mimic the water-splitting step in photosynthesis that generates oxygen (O2) and hydrogen (H2), the latter a highly sought-after renewable fuel that spews nothing but water vapor when burned.1 The apples are oxygen atoms, the pink flowers pyridyls, and the Gerbera daisy was a place where they tried many different additions to the manganese to tune the properties of the molecule.
The memory of this Springy little drawing gave me a strange sense of hope, making me wonder what’s been going on in the field of artificial photosynthesis since then. Turns out, a lot. I followed that bit of hope along the path of my own curiosity, and this is what I found along the way.
Act I: Mimicking the light-dependent reactions for electricity and fuel
Photosynthesis is a performance in two acts—the first a series of light-dependent reactions powered by the sun. Mimicking Act I of photosynthesis is how we got solar panels and devised catalysts to create liquid hydrogen from water.
Energy from the sun
In real photosynthesis, photons from the sun first liberate electrons from chlorophyll molecules. Electrons on the move make electricity. But you can’t even make your hair stand up by rubbing it with a leaf so how did we get to solar panels? Chemistry, physics, materials engineering, and a lot of basic research. Just two weeks ago a team at the Universität Würzburg in Germany published results on a stack of flat molecules that electrons hop along with high efficiency, as if through a wire.23 It could mean getting more bang for our solar buck.
In leaves, the electrons travel through a Rube Goldberg machine called the electron transport chain, storing energy that will then be used in Act II to turn carbon dioxide into carbohydrate. By the time they make that fuel that we love to eat, they’ve already made hydrogen like it was no big deal.
Harnessing the Hindenberg
But it is a big deal! Mimicking the water-splitting step like the flower power molecule I drew above allows us to store the energy in the form of liquid hydrogen fuel. Harvard professor Dan Nocera, perhaps the best known for this work, had already created and sold a company based on his “artificial leaf” catalytic technology many years before I drew that picture in 2017.4 More recently, the Department of Energy had seven “hydrogen hubs” planned, but four are now on the chopping block. It’s a controversial fuel whose safety is saddled with a dark history.
Liquid hydrogen is already used in rocket fuel but mimicking it at a practical scale to meet all of our energy needs requires massive improvements in efficiency, durability, and cost. But scientists and engineers have solved these problems before for things like semiconductors, solar cells, and even electricity itself.
Act II: Mimicking the dark reactions for CO2 sequestration and useful products
In the second act of photosynthesis, known as the dark reactions only because they don’t need light, energy hoarded from the light reactions is used to convert carbon dioxide into carbohydrates. Mimicking Act II of photosynthesis has inspired advances in sequestering carbon dioxide and turning it into other useful materials, including the final frontier: renewable fuel.
Concrete solutions to our carbon problem
Again, like all aspects of artificial photosynthesis, achieving these goals at a practical scale takes massive improvements in efficiency, durability, and cost. In the history of science, thorny problems have often been approached with contests that award large cash prizes for science and engineering solutions. From estimating the longitude of a ship at sea to reverse the epidemic of shipwrecks in the 1700s5 to laying the groundwork for germ theory in the 1800s6, these high-stakes competitions captured the public’s imagination. Even though the public is not as engaged as they used to be, because, Netflix etc., these sorts of competitions still happen. Inspired by the prize that led Charles Lindbergh to be the first to fly non-stop from New York to Paris, the X Prize (xprize.org) has been sponsoring competitions like this for 30 years with numerous concurrent privately funded prizes in the millions.
A recent $20 million carbon dioxide utilization prize was split between two companies, CarbonCure and CarbonBuilt, both of which have adjacent technologies for sequestering carbon dioxide into concrete, turning skyscraper construction into a carbon-negative endeavor.
But to really make a dent in our problems, any solution must be feasible on a massive scale. A current X Prize put an unprecedented $100 million up for grabs for the challenge of showing the ability to remove billions of metric tons of carbon dioxide from the environment without hurting it in any other ways.
Over a thousand teams entered. $1 million Milestone Prizes were given to each of 15 frontrunners after the first year, including teams using kelp forests to sequester carbon, electrochemistry to lower the acidity, and even the equivalent of a giant Tums for the ocean . The ocean is the world’s biggest CO2 sink. The more CO2 can be sequestered in or as other materials, the more of it can be taken in from the air. One team is piggybacking on existing infrastructure like desalination plants to scale up quickly.
The final twenty teams are now in the testing phase. The winner or winners of this competition are scheduled to be announced soon.7 X Prize just released the first 7-minute episode of a docuseries about the competition.8 There’s a lot to love about it and I recommend watching, but I couldn’t help but think if there was more focus on the humans themselves, it could easily be as compelling as The Great British Bakeoff. Why isn’t it on Netflix? You know there has to be all sorts of drama, tension, and even romance both on and between these teams. The humans behind the science are fighting for their livelihoods and for the entire planet. For us. How much higher could the stakes be? What if they could draw as much attention as The Bachelor or America’s Got Talent?
The final frontier
Perhaps the most exciting prospect for the future is to take CO2 from thin air (or water) and turn it directly into renewable fuel. Last fall a team at the University of Michigan funded by the U.S. Army Research Office announced a soup-to-nuts system with huge leaps in efficiency.9 They created light-capturing gallium nitride nanowires so thin that a bundle of a thousand would only begin to reach the width of a human hair. Submerged in the equivalent of seltzer water and dotted with copper, the nanowires soak up light, split water, and convert carbon dioxide to the 2-carbon ethylene, a building block of plastics. If they can string more carbons together they will start to have a real fuel. For now, they’re licensing the technology to a startup called NX Fuels. Again, we’re back to needing great strides in improving efficiency, cost, durability, and scale. But again, we’ve done it before.
Thanks for joining me on this brief learning spree, all of which was inspired by a silly 8-year-old drawing and some flower buds that insisted on bursting out into our world, such as it is. Here’s what the intrepid Forsythia’s been up to in the meantime:
Manganese–Cobalt Oxido Cubanes Relevant to Manganese-Doped Water Oxidation Catalysts. Andy I Nguyen, Daniel L M Suess, Lucy E Darago, Paul H Oyala, Daniel S Levine, Micah S Ziegler, R David Britt, T Don Tilley, J. Am. Chem. Soc. 2017, 139(15), 5579–5587.
Ernst, L., Song, H., Kim, D. et al. Photoinduced stepwise charge hopping in π-stacked perylene bisimide donor–bridge–acceptor arrays. Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01770-7
These molecules are polycyclic aromatic hydrocarbons (PAHs), which don’t have very good PR for human health, so scalability might need to take that into consideration, but a noteworthy advance nonetheless!
Lockheed-Martin bought it more for some flow cell technology associated with the company. Their hydrogen generation technology may have been ahead of its time.
Britain’s Parliament established the Longitude rewards through the 1714 Longitude Act, for whoever could best estimate a ship's longitude at sea, beautifully depicted in Dava Sobel’s book, Longitude: The True Story of a Lone Genius Who Solved the Greatest Scientific Problem of His Time.
The French Academy of Science held a contest for the best study addressing whether or not spontaneous generation—living things emerging from non-living things—was real. The story of Louis Pasteur’s mid-1800s prize-winning adventure and the rivalry with his opponent that captured the public’s attention is beautifully depicted in Carl Zimmer’s New York Times article, Louis Pasteur’s Relentless Hunt for Germs Floating in the Air. Thanks to this achievement, people finally began to accept the link between germs and infectious disease, and acting accordingly.
Interestingly, the billionaire who put up the $100 million from his foundation for carbon capture is the same one who is now working directly to pull back on some of the Department of Energy’s hydrogen hubs.
https://www.xprize.org/prizes/carbonremoval/articles/pulling-co2-out-of-our-oceans-combating-climate-change-through-carbon-dioxide-removal
https://news.umich.edu/in-step-toward-solar-fuels-durable-artificial-photosynthesis-setup-chains-two-carbons-together/




So glad you rediscovered your 8-year-old drawing and shared this wonderful journey with us.
Thank you for sharing! Awesome to see this progress. I realize ethylene itself isn't a fuel, but it is such a great building block chemical - I can imagine easily using well established chemistry to turn it into fuel, or just substituting the solar produced ethylene in everywhere ethylene is currently used. Very exciting!